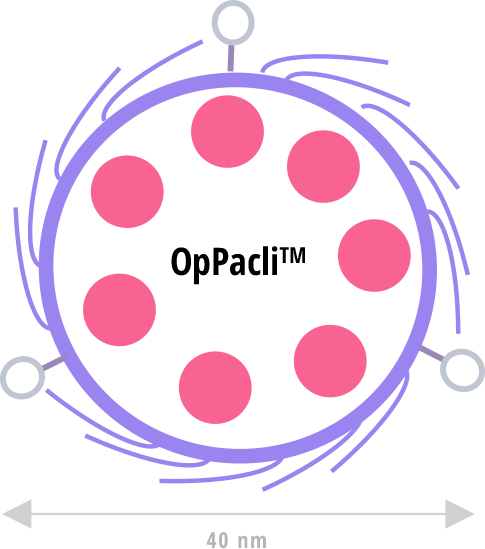
At Pharma in silica, we developed an original silica-made nanocarrier that is likely to reduce the side effects and improve the efficacy of the chemotherapy treatment of solid cancers.
We believe a more ambitious goal is at hand: a true precision chemotherapy. Precision, not as in “personalized medicine” or “cohort-specific immunotherapy”. Precision as in “killing only cancer cells” without maiming the person living with this cancer.
Our wager is that diverse clever additions to the OpPacli™ nanocarrier will generate such precision chemotherapy.
Send us a non-confidential one-pager describing your solution(s). We will talk and make it work, via industrial co-development, grant-supported collaborative research, licensing or acquisition.
For more information on Pharma in silica, visit the company’s web site or send an email.
Pharma in silica arcandf@precision-chemotherapy.com